Details of the Drug
General Information of Drug (ID: DMWR1JC)
| Drug Name |
Carisoprodol
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
carisoprodol; 78-44-4; Isomeprobamate; Carisoprodate; Carisoprodatum; Isoprotane; Isobamate; Isoprothane; Carisoma; Sanoma; Isopropyl meprobamate; SOMA; Somalgit; Atonalyt; Mioratrina; Mioartrina; Flexartal; Skutamil; Miolisodal; Mioril; Apesan; Relasom; Nospasm; Flexal; Arusal; Somanil; Carlsoprol; Carisoprodolum; Domarax; Stialgin; Somadril; Carsodal; Mioriodol; Mediquil; Izoprotan; Isoprotan; Carisol; Caprodat; Calenfa; Diolene; Carlsoma; Carlsodol; Brianil; Flexidon; Flexagilt; Caridolin; Meprodat; Listaflex; Flexagit; Fibrosona; Chinchen; Carsodol
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
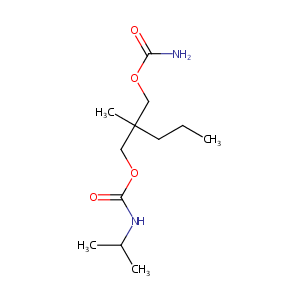 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 260.329 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Carisoprodol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Carisoprodol FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7610). | ||||
| 3 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Lewandowski TA: Pharmacokinetic modeling of carisoprodol and meprobamate disposition in adults. Hum Exp Toxicol. 2017 Aug;36(8):846-853. doi: 10.1177/0960327116672912. Epub 2016 Oct 10. | ||||
| 6 | Association between blood carisoprodol:meprobamate concentration ratios and CYP2C19 genotype in carisoprodol-drugged drivers: decreased metabolic capacity in heterozygous CYP2C19*1/CYP2C19*2 subjects? Pharmacogenetics. 2003 Jul;13(7):383-8. | ||||
| 7 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 8 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 9 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 10 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 11 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 12 | Product Information. Isturisa (osilodrostat). Recordati Rare Diseases Inc, Lebanon, NJ. | ||||
| 13 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
| 14 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 15 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 16 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 17 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 18 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 19 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 20 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 21 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 22 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 23 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 24 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
